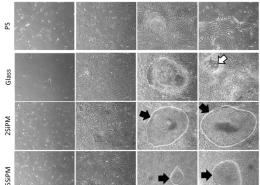
IPFA Award 2019 – Professor Thierry Burnouf, Taipei Medical University
Source: College of Biomedical Engineering
Published on 2019-09-20
Professor Thierry Burnouf, PhD, Vice-Dean of the College of Biomedical Engineering, and Director of the International PhD Program in Biomedical Engineering at Taipei Medical University has been awarded the International Plasma Fractionation Association (IPFA) Award 2019 “in recognition of his exceptional scientific contributions to new plasma fractionation technologies and programmes, and virus inactivation and removal procedures.”
This is the second time that IPFA presents an award to “recognize a person who has made exceptional contributions to the field of plasma collection, plasma fractionation, the manufacturing and provision of plasma derived medicinal products, patient care, safety and transfusion medicine.” Last year the IPFA Award 2018 was presented to Dr. Michael P. Busch, Director, and Professor of Laboratory Medicine at University of California San Francisco, USA.
Professor Burnouf’s research biomedical engineering activities related to processes for the biological therapeutic product industry are at the forefront of the development and industrial implementation of novel plasma fractionation and blood processing technologies. Among his previous achievements in industrial biotechnology are the development of highly-purified plasma derived medicinal products, including coagulation factors and immunoglobulins, which have greatly improved the quality of treatment of patients with bleeding and immunological disorders worldwide. Prof. Burnouf has also contributed to the bioengineering and widespread use of several industrial technologies of virus inactivation and virus removal procedures allowing to ensure the virus safety of blood protein products and other biologicals.
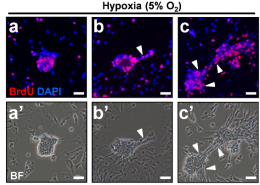
His current field of research interest focuses on bioengineering and bioprocessing of virally-safe human platelet growth factor preparations for regenerative medicine and stem cell therapy. His laboratory has developed procedures ensuring the virus safety of platelet lysates preparations needed for the propagation of human mesenchymal stromal cells for use in cell therapy and regenerative medicine applications. One of the focuses of his laboratory is also the engineering of human platelet products rich in unique mixture of growth factors for treating neurological (Parkinson’s disease, amyotrophic lateral sclerosis, traumatic brain injury, etc.) and ocular disorders.
Professor Burnouf has been a WHO temporary advisor and consultant for the drafting of several Guidelines/Recommendations on (a) “viral inactivation and removal procedures intended to assure the viral safety of human blood plasma products”, (b) “production, quality control and regulation of plasma for fractionation”, (c) “GMP in blood establishments”, as well as a report on “Improving access to safe blood products through local production and technology transfer in blood establishments in developing countries, various “Aide-Memoires” on contract plasma fractionation program and safety of blood products. He is a member of working parties on “Cellular therapies” and “Global Blood Safety” for the International Society of Blood Transfusion.
Dr. Burnouf has published over 250 articles and book chapters related to bioprocessing technologies of plasma and is an inventor of over 20 international granted patent families in the biotechnology field.























-260x185.jpg)















































與連江縣衛生福利局陳美金局長簽署醫療合作備忘錄-260x185.jpg)















期許永續發展成為醫療產業新契機。-260x185.jpg)