TMU leads Taiwan in systemic cell therapies and regenerative medicine


Although TMU triumphed over 20 universities to host Taiwan’s Regeneration Technology Development program, it has many partners in this exciting new field. TMU’s Center for Cell Therapy and Regeneration Medicine cooperates with five U.S. universities and one U.S. company, four Taiwan companies, a Korean university and hospital, two top Japanese universities, and both Hong Kong University and the Duke-NUS Medical School, according to center director Prof. Rita Yen-Hua Huang.
In contrast to controversies surrounding cell therapies elsewhere, Taiwan’s government has supported TMU research into mesenchymal and “small blood” stem cells instead of embryonic stem cells. The nation’s progressive regulatory environment also supports research teams as they develop new applications and technologies.
The center’s work so far ranges from basic research through pre-clinical animal studies, with a triple focus on 1) stem cells and regenerative medicine, 2) use of these techniques in translational medicine, and 3) development of ethical outreach and educational services for industry and society. The translational focus combines the work of doctors, scientists and businesses to find safe and effective new techniques that address unmet medical needs.
The center’s basic research studies niche and embryonic pluripotency, stem cell immune modulation and epigenetics, and nanodrug and cancer stemness. Promising clinical applications are tested with preclinical studies in which stem cells and immune-related mechanisms are being used to treat cancer, pressure ulcers (bedsores), burns and multiple sclerosis.
Center innovations closest to clinical trial stage involve guided bone regeneration in implant surgery and a patch to heal diabetic foot ulcers; Taiwan’s Food and Drug Administration is reviewing a proposal to use small blood cells in dental implants. Prof. Huang noted that this technology is very promising for Taiwan’s aging society, where many elders endure the discomforts of dentures.
In contrast, the field of regeneration therapies is very young; Prof. Huang said that as of 2017, only 55 cell therapy products had been approved on world markets, with Japan providing 4 and the US more than 20. Of two immune-based cancer therapies under development, one uses natural killer cells, a non-stem cell type, and is in Phase 1 trials. The current three-year grant will cover finishing this phase next year, with the next phase tentatively scheduled for 2019. If this trial goes well, the therapy will be offered for industrial development to support the costs of Phase 3 testing. The principal investigator retains patent ownership on behalf of the research team under Taiwan laws, so industrial development can lead to further support for the TMU laboratory and its work.
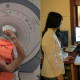
Caption: GTP core laboratory of TMU Center for Cell Therapy and Regeneration Medicine
Caption: The proliferation and migration of germline stem cells (GSCs).
(A, a) The diagram of asymmetric cell division. (b-e) Alkaline phosphatase+ (AP+) stem cell (red color) underwent a series of asymmetric division. The colorless cells were differentiating cells. (B, a) The diagram of symmetric cell division. (b-e) Alkaline phosphatase+ stem cell (red color) underwent a series of symmetric division. (e) Migrating stem cell (arrowhead). (C) AP+ GSCs underwent cell proliferation (BrdU+ cells) under hypoxia (5% O2). The arrowheads indicate GSCs were migrating out from the sphere of stem cells. Photographed by fluorescence microscope (a-c) and phase contrast microscope (a’-c’).
For interviews or a copy of the paper, contact Office of Global Engagement via global.initiatives@tmu.edu.tw.