A Step Beyond CAR-T: Culturing Bispecific Antibodies:
Dr. Kuo-Hsiang Chuang

Associate Professor, Graduate Institute of Pharmacognosy, Taipei Medical University
After building expertise in protein and antibody engineering at Kaoshiung Medical University under Dr. Tian-Li Cheng, Dr. Kuo-Hsiang Chuang jumped at the opportunity to join the TMU family in 2012 at the Graduate Institute of Pharmacognosy. Dr. Chuang says that mentorship under Dr. Cheng was invaluable, both for learning techniques to develop therapeutic bi-specific antibodies and for developing a professional and productive laboratory culture.
“I learned a lot about the scientific method and good attitude from my teacher. [Dr. Cheng] taught us that you need to have good friendships with your colleagues, and smart experimental methods,” said Dr. Chuang, “And work hard. Work very hard!”
T-cells are the most powerful cells of the body’s immune system. They are responsible for eliminating invaders or abnormal cells, but aren’t very good at recognizing cancer on their own. At the cutting edge of immune antibody treatments, CAR-T therapies have extended the lives of thousands of patients by modifying the body’s own immune cells to attack and destroy cancer. CAR-T treatments indeed look promising; products like Kymriah and Yescarta have been a game-changer for treating leukemia and b-cell lymphoma, and globally there are presently close to 350 ongoing CAR-T clinical trials.
In CAR-T therapy, T-cells are harvested from a patient or donor’s blood, given new genes using a retrovirus so they can grow a specific receptor (a chimeric antigen receptor, or CAR), and are reintroduced into the patient – now able to attack a specific target like cancer. But engineering CAR-T treatments is a complicated and time-consuming process; it is expensive and not without risk.
According to Dr. Kuo- Hsiang Chuang, besides costing upwards of half-a-million dollars per treatment and carrying the risk of causing deadly cytokine release syndrome, CAR-T treatments themselves can become carcinogenic if the CAR gene is unintentionally introduced into cancer cells – effectively conferring resistance to a patient’s cancer. On top of that, the process takes around 30 days, time that a cancer patient may not have.
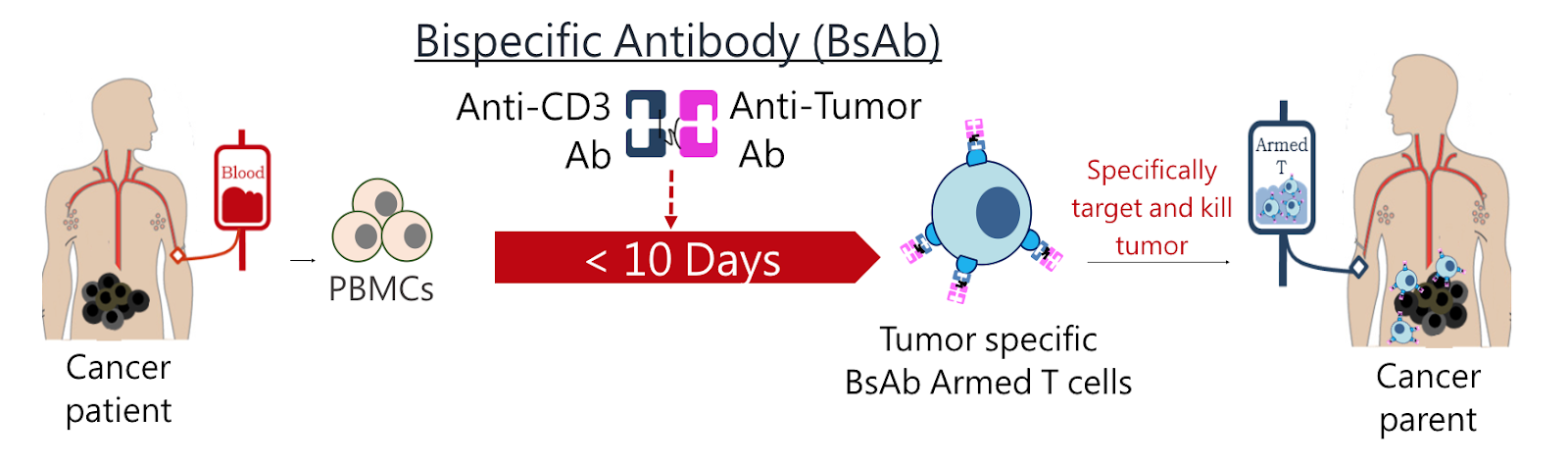
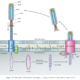
With these problems with CAR-T therapy in mind, Dr. Chuang developed a novel method of culturing antibodies that he says is faster, cheaper, and safer than traditional CAR-T methods. Dr. Chuang’s strategy is to use bi-specific antibodies (BsAbs) that can recognize two antigens at the same time.
Specific BsAb treatments can be built a bit “like Lego”, says Dr. Chuang. “We can reassemble the protein or antibody depending on our needs. One arm [of the BsAb] can target the tumor, and the other arm can target something to help you kill the tumor effectively.”
As with CAR-T, blood cells were first harvested from patients. Then in Dr. Chuang’s one-step process, BsAbs that bind to human T cell’s CD3 receptor and an anti-PSMA (prostate specific membrane antigen) agent were added to the culture medium. These BsAbs were able to differentiate and expand human CD3 positive T cells, and when introduced into mice, accumulated at tumor sites, leading to tumor cell death for two types of cancer.
Because Dr. Chuang’s BsAb culturing method does not require retro-virus based gene transfection, it will not unintentionally confer treatment resistance to cancer cell, and because it does not require a laborious incubation process, it is much cheaper and faster. Instead of a 30-day wait, a patient-specific treatment could be ready in less than 10.
With his study showing promise for targeting EGFR (epidermal growth factor receptor) and PSAM positive cancers, Dr. Chuang and team are looking at using different particular antibody building blocks to precisely target other cancers or autoimmune diseases like rheumatoid arthritis and type 1 diabetes.
The future of Dr. Chuang’s non-viral bispecific antibody culture method looks bright. With help in part from TMU’s Office of Business Development in providing support services for financing, facilitating tech transfers, academia-industry liaising, and making patent applications, the team have already been approached for research collaborations and development of new bispecific antibody therapies with industry players.
For interviews or a copy of the paper, contact Office of Global Engagement via global.initiatives@tmu.edu.tw.