Breakthroughs in Niche Microenvironments and Systemic Safety Therapeutic Cell Product Development
Dr. Yen-Hua Huang

- Distinguished Professor and Dean, Office of Research and Development
- Executive Director, TMU Center for Cell Therapy and Regeneration Medicine
- Director, Good Tissue Practice Lab.
- Professor, International Master/Ph.D. Program in Medicine, College of Medicine
- Professor, International Ph.D. Program for Cell Therapy and Regeneration Medicine
Winner of a number of prestigious research awards, including the MOST Outstanding Young Scientist Research Grant and the Outstanding Basic Research Award for Cancer Medicine, Dr. Huang holds patents for two stem cell processes with another to be awarded this year.
Two-time winner of Taiwan’s Ministry of Science and Technology Outstanding Young Scientist Incubation Research Grant and holder of two stem cell related patents, Dr. Rita Yen-Hua Huang has made quite a name for herself as a star researcher. After 20 years of studying embryonic pluripotency, cancer stemness and cell therapy products, Dr. Huang brings her expertise to TMU Research Center for Cell Therapy and Regeneration Medicine and the GTP Laboratory to focus on the development of systemic safe and effective cell therapy products. Dr. Huang and her team are considered global leaders in the field and have developed a novel culture method to generate the stem cells with systemic safety and efficacy both in research and clinical treatments.
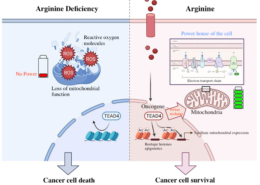
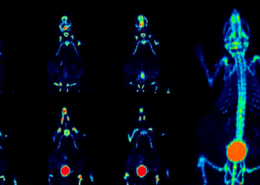
Niche microenvironment will affect cell fate dominantly. In cancer study, Dr. Huang looked at the critical role of inflammatory microenvironment in tumor pluripotency and recurrence in patients with liver cancer with and without Hepatitis B. Because the Hep-B virus persists in the body long-term, patients display higher levels of the inflammatory IL-6 cytokine. The researchers found that in patients with HBV – and thus higher IL-6 levels – cancers were more likely to spread.
“If the microenvironment changes, stem cells will transform to cancer stem cells, or cancer cells will get reprogramming to express stemness then migrate easily, metastasizing and causing drug resistance. So, treatment fails or a patient relapses early,” says Dr. Huang. This has important implications for treatment because patients found to have high levels of IL-6 can be given IL-6 neutralizing antibodies – or even aspirin, which is anti-inflammatory – and should have improved prognoses.
As scientists look to stem cells for research and new clinical treatments, understanding how niche microenvironment affects stem cell growth and differentiation is becoming critical. To insure they keep their original characteristics – and to avoid tumor promotion risk – cells must undergo repeated passages with symmetrical division to produce quantities necessary for large-scale use.
This is where some groups have run into problems. Osiris Technologies, for example, had promising early successes with Prochymal, an IV administered mesenchymal stem cell (MSC) product designed to treat children whose bodies reject bone marrow transplants that was cleared for use in Canada and New Zealand. Although Prochymal successfully completed Phase 1 and Phase 2 clinical trials, a large-scale Phase 3 trial unfortunately failed.
“The cells may differentiate and age,” suggests Dr. Huang. “There is devil in the cell preparation. They had to generate so many cells for a lot of patients. They lost cell quality, the epigenetics changed and the cells differentiated. ” Osiris Technologies offloaded the product in 2013.
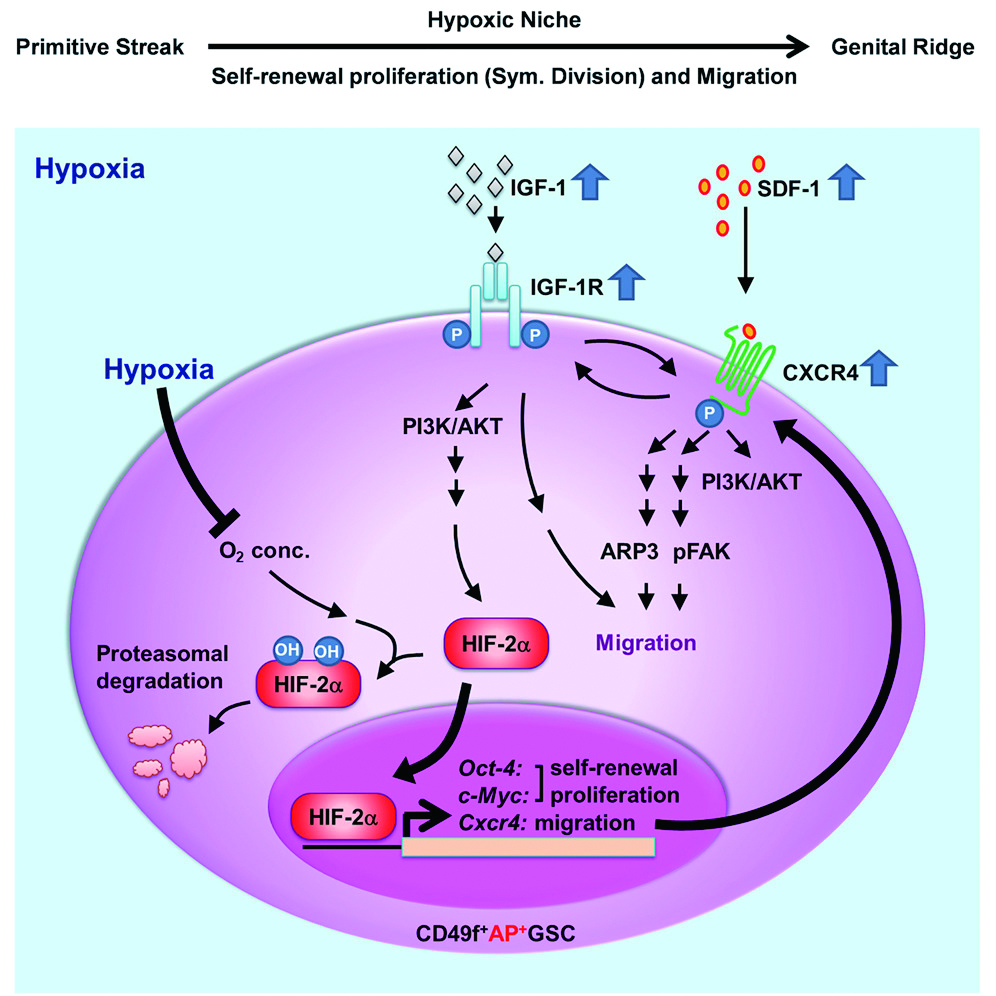
Seeing the weakness of corporate R&D programs, Dr. Huang began working on growing large numbers of stem cells without having them differentiate and being aged. Looking at embryonic germline stem cells, she and her team identified specific niche micro-environmental factors that play a critical role in the symmetric division and migration of embryonic germline stem cells.
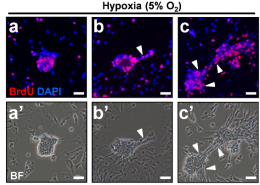
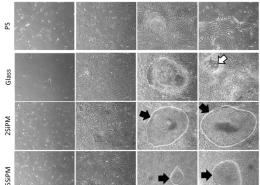
In their study published in Stem Cell Reports, the team cultured pluripotent germline cells in a unique serum-free culture platform. By manipulating oxygen levels and monitoring crucial stemness protein levels, they were able to demonstrate that hypoxia cooperated synergistically with IGF-1R signaling to regulate stem cell symmetric division, proliferation, and cell migration.
Generating identical stem cells may be a delicate and difficult to control process, but now says Dr. Huang, “We know what environment and when the signal transduction can stimulate embryonic stem cells and MSCs to go into symmetric division. Now we can generate a lot of symmetrically dividing MSCs for clinical use.”
The team received 2019 The 16th National Innovation Award for their work, and Dr. Huang’s third patent is expected later this year. “We can have a really beautiful SOP. We control cell quality, we know cell safety, we know the genetics, the transcriptome data, single cell sequencing, before and after cell responses to different kinds of injury on microenvironment. We can generate a lot of symmetrically dividing and systemic safety mesenchymal stem cells for clinical use.”
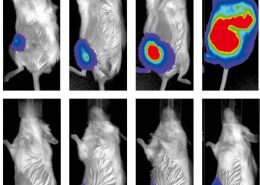
With a better understanding of cell culture protocols stemming from these discoveries, the allogeneic safety clinical grade MSCs from specific placenta tissues can now be produced in large numbers at TMU’s GTP lab. Under her special protocol, cells retain stemness, and can exceed 25 successful passages (cells in most other methods die off after 10). Importantly for patients, the cells are systemic safety and free of tumor risk which do not initiate or promote tumor growth. The allogeneic MSC cell therapy clinical trial targeting COVID-19 acute respiratory distress syndrome (ARDS) now is submitting to FDA for review.
Did you know? |
| Researchers trying to generate stem cells for use in clinical settings run into two main difficulties. One is generating a useful amount of cells; over repeated rounds of passage (growing cells over generations), the primary cells will differentiate, age and eventually die. Another is that cells’ “stemness” (their ability to self-renew, differentiate, and proliferate) itself is not always a good thing; under certain conditions stem cells can transform into cancer cells, or to promote tumor cell proliferation, or cancer cells can get reprogramming ability to develop stemness themselves – allowing them to easily metastasize and develop drug resistance. Targeting niche microenvironment is always the critical point for both the cancer treatment (tumor niche) and the systemic safety stem cell product development (culture niche). |
For interviews or a copy of the paper, contact Office of Global Engagement via global.initiatives@tmu.edu.tw.















-260x185.jpg)















































與連江縣衛生福利局陳美金局長簽署醫療合作備忘錄-260x185.jpg)















期許永續發展成為醫療產業新契機。-260x185.jpg)