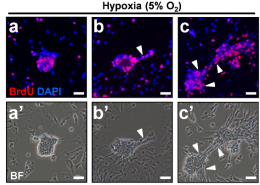
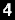The world’s first endometrial cancer test reagent, MPap® DNA Methylation Detection Kit is launched by TMU spin-off Guzip Biomarkers Corporation
Source: Office of Development
Published on 2021-11-08
GMU’s Student Service Society was recognized with three awards at the 2017 Young Overseas Peace Mission Recognition and Sharing Conference. The Swaziland, Cambodia and Nepal global medical services teams won the gold, silver and bronze awards respectively.
MPap® combines the conventional cervical smear sampling with DNA methylation detection technology, which successfully passed the Innovative Medical Device Review of the Food and Drug Administration, Ministry of Health and Welfare in August 2021, indicating that the endometrial cancer in Taiwan has now entered the novel molecular diagnostic technology era.
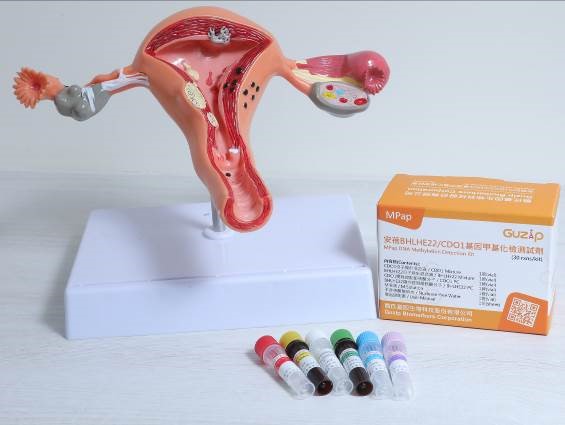
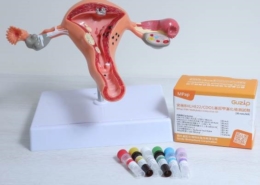
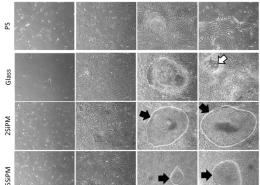
MPap® DNA Methylation Detection Kit
MPap® can detect multiple methylated genes in one time. It provides physicians with reference for diagnosis and treatment, reduces the inconvenience and risk of invasive diagnostic testing, implements early detection and treatment to safeguard women’s health. MPap® has obtained patents in many countries, including Taiwan, Japan, Russia, South Korea, South Africa, etc.
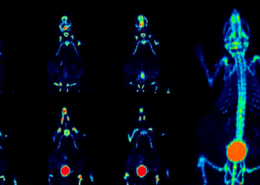
The incidence rates of endometrial cancer have been rising and outnumbered cervical cancer and ovarian cancer, making it the most common gynecological cancer. The most common symptom of endometrial cancer is abnormal uterine bleeding. However, there are many causes of bleeding. In clinical practice, physicians need to use invasive endometrial sampling for cancer diagnosis. Nonetheless, invasive diagnostic testing causes discomfort and poses other risks of bleeding.
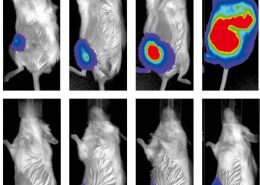
According to the analysis of Taiwan’s National Health Insurance Research Database, the conventional diagnosis of an endometrial cancer patient required at least 28 times invasive endometrial sampling. However, by using MPap®, the clinical diagnosis efficiency can be increased by 3 times, improving the quality of diagnosis.

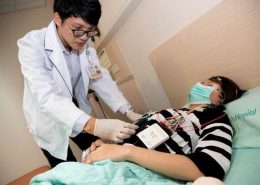
Dr. Hung-Cheng Lai, the inventor of MPap® DNA Methylation Detection Kit and physician at Shuang Ho Hospital
The inventor of MPap® DNA Methylation Detection Kit, Dr. Hung-Cheng Lai, is a professor at the School of Medicine of Taipei Medical University and a physician of the Department of Obstetrics and Gynecology at Shuang Ho Hospital. The product based on his invention is the world’s first DNA methylation for cervical cancer screening. . In 2018, he co-founded Guzip Biomarkers Corporation with Dr. Polly Lin (林淑娟博士), a biologist with years of experience in business development and operational management, and applied their understanding of patient needs and clinical insights to reduce the cost and time of trial and error for medical devices entrepreneurship.
Dr. Polly Lin, CEO of Guzip Biomarkers Corporation, said that the intention of starting a business is to provide scientific applications to the women’s healthcare industry. She is very glad that their three years of efforts in developing a diagnostic tool for endometrial cancer has been approved by the Taiwan Food and Drug Administration (TFDA), providing the industry with the pioneering product. In the future, they will actively promote the products to major medical centers and clinics to benefit more women in need.






















-260x185.jpg)















































與連江縣衛生福利局陳美金局長簽署醫療合作備忘錄-260x185.jpg)















期許永續發展成為醫療產業新契機。-260x185.jpg)